 |
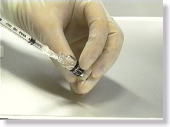
<Photo. 01>
"Karton-CI" --- the clear agent on the right
"Karton-CII" --- the yellow agent on the left
|
|
|
 |
<Photo. 02>
Drawing in 0.2 ml of "Karton-CI" into a syringe. It is, in fact, more glutinous than its appearance, but continue to suck in the agent without worrying about the air that goes in with it.
|
|
|
 |
<Photo. 03> Following Karton-CI, draw in 0.1 ml of "Karton-CII" into the
same syringe. "Karton-CII" is a smoother agent, so it is
easier to suck in. Try not to mix the two agents inside till you
are ready for its use. If you mix them too soon, they'll begin to
gel as seen in the photo 04 below. When the time comes for its use,
suck in some air and shake the syringe to mix them. When the agents
are well mixed, then get the air out of the syringe. |
|
|
 |
<Photo. 04>
This photo shows how gelation is taking place. Please use the mixture
within approximately 5 minutes at latest. |
|
|
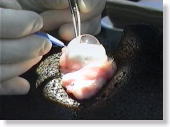 |
<Photo. 05>
Your practice begins with, at first, making a side port around the equator
of the sclera. Observe the enlarged photo below, also. |
|
|
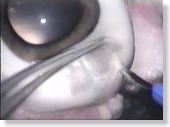 |
<Photo. 06>
Cut at a stroke by using a stab knife. Note: If you click the photos,
even larger pictures will be shown.
|
|
|
 |
<Fig. 01>
Through the opening you've just made, insert a blunt needle on the syringe
with Karton-C (the mixture of CI and CII) into the vitreous body. |
|
|
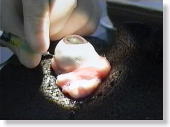 |
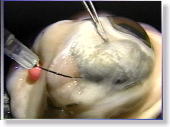
<Photo. 07>
This is the actual photo of the process. Observe the photo below,
too.
|
|
|
 |
<Photo. 08>
This is at the same process shown from a different angle.
|
|
|
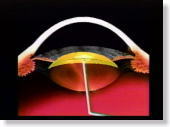 |
<Fig. 02>
Steadily advance the needle toward the pole of the posterior capsule and
pierce the posterior capsule. Then, advance the needle until it is
right under the center of the anterior capsule and then inject Karton-C
evenly into the space. |
|
|
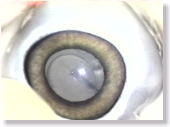 |
<Photo. 09>
The photo shows how Karton-C should spread in the space beneath the anterior
capsule. Limit the amount of injection to 0.2 ml and avoid overdosing. |
|
|
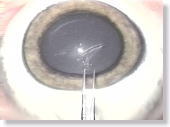 |
<Photo. 10>
The anterior capsule flaps of an untreated pig's eye tend to be very thick
and sticky, and are quite different from human's, so it can hardly be a
practical training model to perform a CCC on. |
|
|
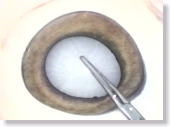 |
<Photo. 11-a>
Here, a CCC is being performed with forceps on the gelled anterior capsule.
The fragility of the anterior capsule flaps approximates that of
human's, so they can easily be turned over. Furthermore, the tissues beneath
the anterior capsule have ideally gelled and opacified, resembling those
of a human cataract. Also see the photos in 11-b below.
|
|
|
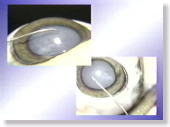 |
<Photo. 11-b>
These are photos from different angles.
|
|
|
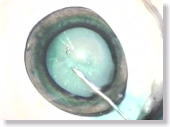 |
<Photo. 12-a>
ICG (Indocyanine green) has been injected into the anterior chamber to
make it more visible. As seen in this photo, the anterior capsule
stained green is clearly more identifiable. Also see the photos in
12-b below.
|
|
|
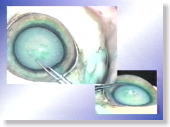 |
<Photo. 12-b>
Photos from other angles.
|
|
|
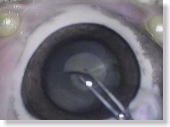 |
<Photo. 13>
When it is stained with trypan blue as in this photo, the status of the
anterior capsulotomy is much more clearly revealed than when stained with
ICG. (However, that of a human cataract is, in fact, much less identifiable.)
|
|
|
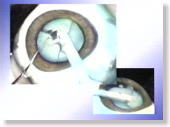 |
<Photo. 14>
The hardness of the Karton-nucleus is close to that of a soft human cataract,
which enables surgeons to easily practice nucleus fragmentation. |
|
|
 |
<Photo. 15>
This is "Karton-N," a clear, vermilion reagent. |
|
|
 |
<Photo. 16>
This is the status of Karton-N after having dipped in water for over 5
minutes. Its color has turned yellow and solidly gelled. |
|
|
 |
<Photo. 17>
To prepare for the next step, perform a CCC on a butchered pig's eye with
a PEA machine as it is done in the usual manner. |
|
|
 |
<Photo. 18>
When the lens is empty, apply viscoelastic material into the anterior chamber,
injecting it in a doughnut shape onto the iris root area only, with caution
of collapsing the anterior chamber. (Try not to inject into the pupillary
area, nor into intralenticular space.) Next, through the side port, inject
Karton-N into the central pupillary area and the intralenticular space
very slowly and cautiously, which process is the most careful point to
attend to.
|
|
|
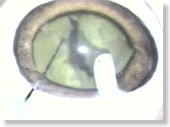 |
<Photo. 19>
After over 10 minutes passed, a fully solidified Karton-nucleus is formed,
on which the 'divided & conquer' technique can also be practiced with
a spatula.
|
|
|
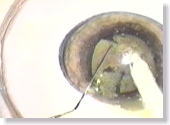 |
<Photo. 20>
Similarly, the 'phaco chops' can be conducted with a hook as well. |
|
|